OUR PIPELINE
Our program focuses on using antibody therapies to fight paediatric cancer. Our lead candidate has completed Phase II trials and is pending further trials and regulatory submissions
Immunotherapy for Neuroblastoma
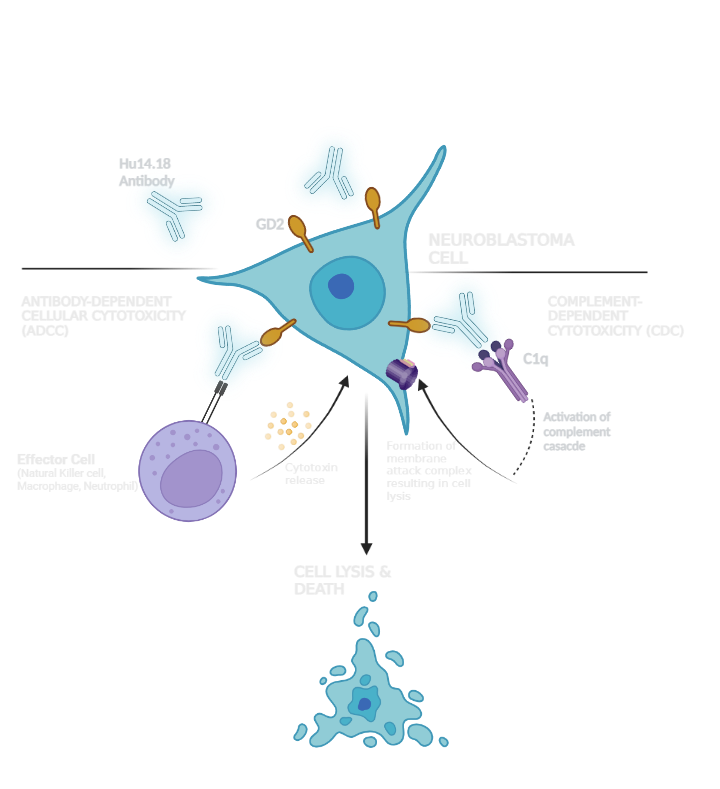
Our lead candidate is an antibody immunotherapy that targets and kills neuroblastoma cells. Immunotherapies involve leveraging the body's immune system to fight cancer. Neuroblastomas abundantly express a molecule called GD2, which inhibits recognition of cancer cells by immune cells, allowing the cancer to evade destruction by the body's immune system1.
Current immunotherapies use antibodies to target and bind to the GD2 molecule, allowing the recruitment of natural killer cells, macrophages and neutrophils to selectively destroy tumour cells2.
GD2 is also highly expressed on small cell lung cancers, osteosarcomas, gliomas and most melanomas3. Anti-GD2 antibodies may also show potential in treatment of these cancers.
Our Program
Our lead candidate, Hu14.18K322A (Hu14.18), is a humanised, anti-GD2 antibody for the treatment of neuroblastoma. This product is derived, in part, from research originally performed at St. Jude Children’s Research Hospital and licensed to Renaissance Pharma Ltd.
Hu14.18 was shown to be tolerable and clinically active in patients with relapsed neuroblastoma 4.
Recent advances in neuroblastoma treatment have revealed that combining anti-GD2 antibodies with chemotherapy from the induction of treatment can enhance patient response and overall survival rates 5,6,7.
When combined with induction chemotherapy in a group of newly diagnosed, high-risk patients, Hu14.18 led to an overall survival rate of 86.0%7, with all patients showing evidence of clinical benefit. Previously, despite intensive polymodal treatment, nearly half of all patients with newly diagnosed high-risk neuroblastoma did not survive.
As a result of this strong Phase II data, Hu14.18 remains our primary development candidate and is pending advancement to further trials and regulatory submissions/discussions with major regulatory agencies.
Image created with BioRender.com
Our Science
The structure and cell line expression of Hu14.18 is different from the existing antibody treatments available for neuroblastoma. It has been designed to potentially reduce immunogenicity, increase cell kill activity and limit off-target effects to provide a treatment that will improve efficacy and tolerability in patients.
Hu14.18K322A (Hu14.18)
…is 98% human, conferring potential for reduced immunogenicity and therefore improved tolerability compared with chimeric anti-GD2 antibodies
…is derived from a cell line with lower glycosylation of the evolved antibody, which results in higher levels of cell kill (antibody dependent cell cytotoxicity, ADCC) activity, and thereby a clinical benefit through enhanced anti-tumour activity.
…is produced with a point mutation to reduce complement activation and therefore pain associated with anti-GD2 binding to peripheral nerves. This enables higher antibody dosing and may provide benefits in tolerability.
Clinical Trials
NB2012 (NCT01857934)
Improved Outcome in Children With Newly Diagnosed High-Risk Neuroblastoma Treated With Chemoimmunotherapy: Updated Results of a Phase II Study Using Hu14.18K322A. Furman, W.L., McCarville, B., Shulkin, B.L., Davidoff, A., Krasin, M., Hsu, C.W., Pan, H., Wu, J., Brennan, R., Bishop, M.W. and Helmig, S., 2022.Journal of Clinical Oncology, 40(4), pp.335-344.
The Phase II NB2012 study, led by St Jude, aimed to illuminate whether addition of the novel antibody Hu14.18K322A (Hu14.18) to a standard induction chemotherapy regimen is safe and whether it improves clinical response in high-risk neuroblastoma patients compared to those receiving chemotherapy only.
The study found that the treatment was well-tolerated, with a 3-year event-free survival rate of 73.7% and an overall survival rate of 86.0%. On completion of the induction chemotherapy regimen, 97% of patients had a partial response or better.
Renaissance Pharma has licensed the antibody Hu14.18 from St. Jude and it is now pending further regulatory submissions and trials.
SJGD2NK & PATA (NCT00743496)
A pilot trial of humanized anti-GD2 monoclonal antibody (Hu14.18K322A) with chemotherapy and natural killer cells in children with recurrent/refractory neuroblastoma. Federico, S.M., McCarville, M.B., Shulkin, B.L., Sondel, P.M., Hank, J.A., Hutson, P., Meagher, M., Shafer, A., Ng, C.Y., Leung, W. and Janssen. 2017. Clin Cancer Res 23(21):pp.6441-6449.
The Phase I SJGD2NK study in patients with recurrent or refractory melanoma aimed to determine tolerability and safety of Hu14.18K322A (Hu14.18) in combination with natural killer (NK) cell treatment and chemotherapy.
SJGD2NK determined maximum tolerable dose of the anti-GD2 humanised antibody Hu14.18 and showed Hu14.18 can be combined with three standard chemotherapy regimens. All patients had clinically meaningful responses and none had cancer that progressed.
This trial helped form the basis for advancement into Phase II, recognising the potential of Hu14.18 as a possible avenue for treatment of neuroblastoma.
Pre-existing Antitherapeutic Antibodies Against the Fc Region of the Hu14.18K322A mAb are Associated with Outcome in Patients with Relapsed Neuroblastoma. Goldberg JL, Navid F, Hank JA, Erbe AK, Santana V, Gan J, de Bie F, Javaid AM, Hoefges A, Merdler M, Carmichael L, Kim K, Bishop MW, Meager MM, Gillies SD, Pandey JP, Sondel PM. J Immunother Cancer. 2020 Mar;8(1):e000590.
This Phase I study sought to evaluate whether the body makes antibodies against the Hu14.18K322A (Hu14.18) antibody. This type of response can limit the tolerability and efficacy of antibody immunotherapies.
The PATA study found that of patients who had not been treated with Hu14.18, 25% had pre-existing antibody responses to Hu14.18. This antibody response did not counteract the benefits of Hu14.18 treatment. In fact, patients who displayed this response may respond better to treatment. This trial may help optimise the design and development of future antibodies to maximise clinical benefit.
GD2NK (NCT01576692)
Phase I trial of a novel anti-GD2 monoclonal antibody, Hu14.18K322A, designed to decrease toxicity in children with refractory or recurrent neuroblastoma. Navid, F., Sondel, P.M., Barfield, R., Shulkin, B.L., Kaufman, R.A., Allay, J.A., Gan, J., Hutson, P., Seo, S., Kim, K. and Goldberg, J., 2014. Journal of clinical oncology, 32(14), p.1445.
GD2NK was a safety & feasibility study in children with refractory or relapsed neuroblastoma. The primary aim was to determine response and toxicities to the Hu14.18K322A (Hu14.18) antibody with and without natural killer (NK) cell treatment when given repeated cycles of a standard chemotherapy regimen.
GD2NK found that chemotherapy in addition to Hu14.18, cytokines and NK cell treatment resulted in a clinically meaningful response worthy of further study.
References
1. Majzner R, 2022, Anti GD2 Antibody Therapy - an important approach in the treatment of neuroblastoma, Neuroblastoma Parent Global Symposium 2022 [Online]. Available from: https://www.youtube.com/watch?v=cSeN8-X5vQo [Accesssed 25 February 2023]
2. Furman, W.L., 2021. Monoclonal antibody therapies for high risk neuroblastoma. Biologics: Targets and Therapy, pp.205-219.
3. Nazha, B., Inal, C. and Owonikoko, T.K., 2020. Disialoganglioside GD2 expression in solid tumors and role as a target for cancer therapy. Frontiers in Oncology, 10, p.1000.
4. Federico SM, McCarville MB, Shulkin BL, et al: A pilot trial of humanized anti-GD2 monoclonal antibody (hu14.18K322A) with chemotherapy and natural killer cells in children with recurrent/refractory neuroblastoma. Clin Cancer Res 23:6441-6449, 2017 1.Federico SM, McCarville MB, Shulkin BL, et al: A pilot trial of humanized anti-GD2 monoclonal antibody (hu14.18K322A) with chemotherapy and natural killer cells in children with recurrent/refractory neuroblastoma. Clin Cancer Res 23:6441-6449, 2017
5. Menard C, Martin F, Apetoh L, Bouyer F, Ghiringhelli F. Cancer chemotherapy: not only a direct cytotoxic effect, but also an adjuvant for antitumor immunity. Cancer Immunol Immunother. 2008;57(11):1579–1587. doi:10.1007/s00262-008-0505-6
6. Mody, R., Alice, L.Y., Naranjo, A., Zhang, F.F., London, W.B., Shulkin, B.L., Parisi, M.T., Diccianni, M.B., Hank, J.A., Felder, M. and Birstler, J., 2020. Irinotecan, temozolomide, and dinutuximab with GM-CSF in children with refractory or relapsed neuroblastoma: a report from the Children’s Oncology Group. Journal of Clinical Oncology, 38(19), p.2160.
7. Furman, W.L., McCarville, B., Shulkin, B.L., Davidoff, A., Krasin, M., Hsu, C.W., Pan, H., Wu, J., Brennan, R., Bishop, M.W. and Helmig, S., 2022. Improved outcome in children with newly diagnosed high-risk neuroblastoma treated with chemoimmunotherapy: updated results of a phase II study using hu14. 18K322A. Journal of Clinical Oncology, 40(4), pp.335-344